Treating incontinence via bladder regeneration
The BladderCell technology platform is the only one known with these patented or patent pending bioelectric signaling sequences
The original patent pending BladderCell device design has these primary components to its candidate therapy.
1. Bioelectric signal sequences for recruiting (via SDF1 and PDGF), proliferating and differentiating stem cells into bladder muscle.
2. Bioelectric signal sequences communicating with the nervous system for neuromodulation control of bladder.
3. Bioelectric signals for controlling release of follistatin, tropoelastin, EGF, HGF and other proteins for regenerating bladder function.
4. Micro infusion pump for repeat deliveries of BC-15 bladder regeneration composition.
5. Fifteen component BC-15 bladder regeneration composition comprised of muscle stem cells, growth factors, exosomes, micro RNA gel, PRF, amniotic fluid, selected alkaloids, nutrient hydrogel, follistatin, SDF1, oxygenated nanoparticles and bladder matrix.
Effect of Electroacupuncture on Urinary Leakage Among Women With Stress Urinary Incontinence: A Randomized Clinical Trial.
The effect of injectable platelet rich fibrin as a nonsurgical treatment of the female stress urinary incontinence.

Leonhardt MicroStimulator II
Leonhardt Implantable Micro Stimulator Option


BladderCell™
MicroImplant Stimulator for Improving Bladder Function via Bioelectric Controlled Protein Expressions
- Only stimulator to control these protein expressions for these purposes….
- SDF1 and PDGF for stem cell homing.
- Klotho, IGF1, Follistatin, LIM Muscle S100a for muscle regeneration.
- Sonic hedgehog, LIM muscle, IGF1 for nerve connection regeneration.
- Full suite of signals for inflammation reduction including klotho.
- Tropoelastin for increasing elasticity.
- COL17A1 for rebuilding tissues.
OF THE 25 MILLION ADULT AMERICANS SUFFERING FROM SOME FORM OF URINARY INCONTINENCE, 75-80% OF THOSE ARE WOMEN.
BladderCell via a combination of patented bioelectric signaling sequences to control regenerative protein expressions is harnessing natural healing regenerative processes in developing combination devices and methods for voiding dysfunction, including decompensated bladder and overactive bladder. For mild to moderate cases the stimulation is non-invasive and infusions, if used, are via standard circulatory IV infusion pump means. For highly severe cases this includes a patented implantable stimulator and re-fillable micro-infusion pump which is re-filled daily with the BC-15 bladder regeneration composition that slowly infuses into the bladder to accomplish total regeneration over time. This proprietary BC-15 composition includes stem cells, myoblasts, exosomes, matrix and a host of other support factors.
Our patented SDF1 and PDGF signals control stem cell homing to the bladder. Our kltoho, follistatin, tropoelastin and COL17A1 signals regenerate muscle, urothelium and restore elasticity. Our IGF1 and sonic hedgehog signals regenerative nerves.
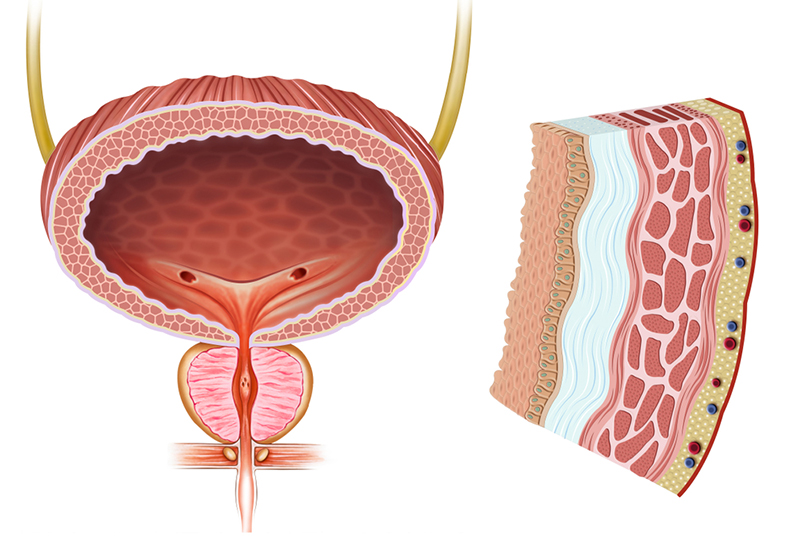
The properly functioning urinary bladder is composed of a compliant muscular wall, effective neuromuscular connections, and a highly specialized urothelial layer. In dysfunction often all these key contributors to healthy normal bladder function are compromised in a deteriorated state. BladderCell is the only known therapy under development attempting to target ALL
Leonhardt Ventures is registered as Leonhardt Vineyards LLC a State of California LLC since 2005 DBA Leonhardt Ventures DBA Filing Document No. 2013239458 Los Angeles County 11/20/13.





