DEVELOPMENT PIPELINE
HEART AND CARDIOVASCULAR
BRAIN
COSMETIC & REPRODUCTIVE HEALTH
CLICK HERE TO VIEW LAUNCHING STARTUPS/LTPs
MAJOR ORGAN REGENERATION
CANCER TREATMENT
Accelerator Graduation Criteria = Complete first in human study (usually 10 to 30 patients) of product near final to go to commercialization addressing a primary target market.
Accelerator Membership Agreement – Click Here
Heart & Cardiovascular
Click Here
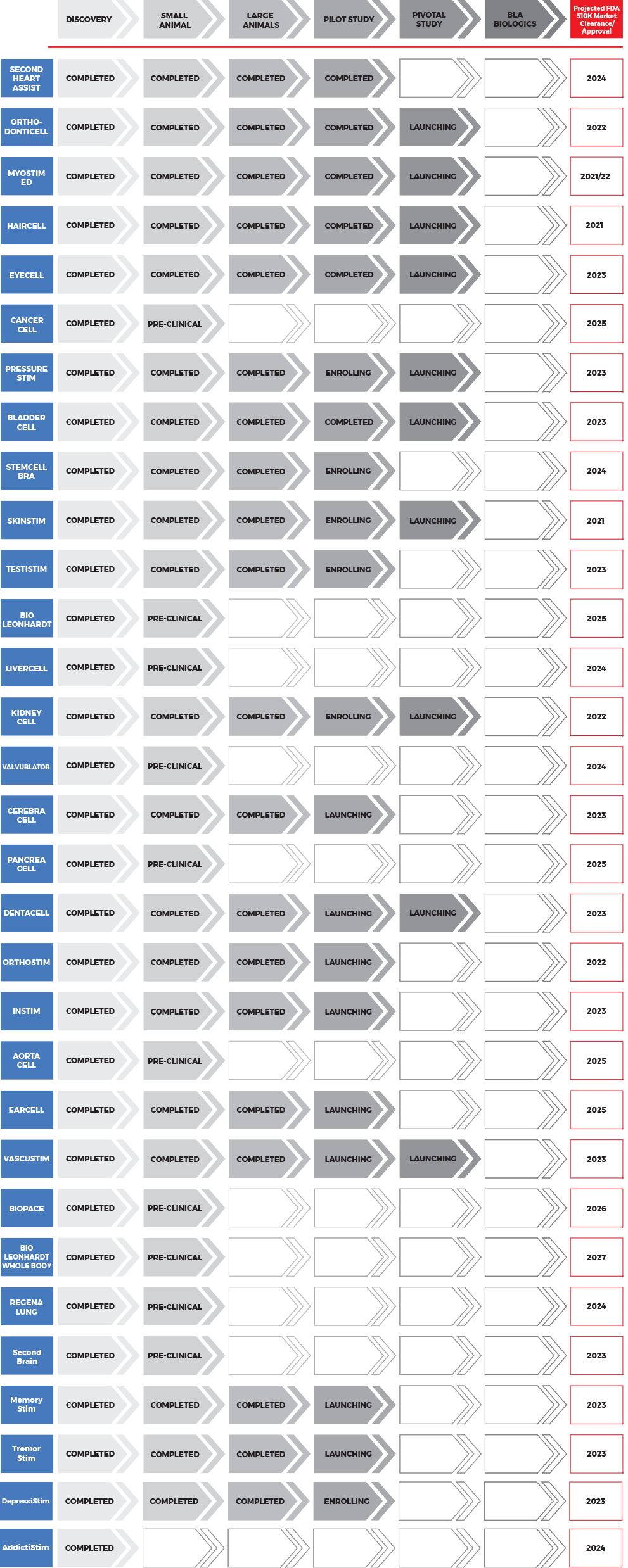
1. Second Heart Assist, Inc. – Graduated January 2022 (graduated early based on only 4 clinical cases)
2. PressureStim – Graduating 4Q 2022
3. Valvublator – Graduating 2023
4. Vascustim – Graduating 2023 (completed 7 pts previously in Mexico)
5. BioLeonhardt – Graduating 2024
6. AortaCell – Graduating 2025
7. BioPace – Graduating 2025
8. VibroCell – Graduating 2024
Brain
Click Here
- DepressiStim – Graduating 1H 2023
- MemoryStim – Graduating 1H 2023
- AddictiStim – Graduating 2H 2023
- CerebraCell – Graduating 2024
- Second Brain – Graduating 2024
- SpineStim – Graduating 2024 (completed pilot study already)
- TremorStim – Graduating 2024
Cosmetic & Personal Care
Click Here
- SkinStim – Graduated January 2022
- HairCell – Graduated January 2022
- ErectiStim – Graduated January 2022
- OrthodontiCell – Graduated January 2022
- KlothoYears – Graduating 1Q 2023 (completed pilot study already)
- Stem Cell Bra – Graduating 1Q 2023 (completed pilot studies already)
- ImplantStim – Graduating 1H 2023 (completed pilot cases already)
- TestiStim – Graduating 1H 2023 (completed pilot case already)
- BreatheStim – Graduating 1H 2023 (completed pilot cases already)
- ArchStim – Graduating 2023
- DentaCell – Graduating 2024
Major Organ Regeneration
Click Here
-
- BladderCell – Graduated January 2022 (pilot clinical studies nearly complete)
- KidneyCell – Graduating 4Q 2022 (pilot clinical studies nearly complete)
- EyeCell – Graduating 3Q 2023 (completed pilot studies already with Dr. Chaikin and Dr. Kondrot)
- OrthoStim – Graduating 2023
- LiverCell – Graduating 2025
- PancreaCell – Graduating 2025
- EarCell – Graduating 2025
- InStim – Graduating 2024 (pilot case already completed)
- BioLeonhardt Whole Body* (full body regeneration chamber) – Graduating 2026
*BodStim stand alone spin out product and startup of BioLeonhardt Whole Body will graduate out in 4Q 2022 or 1H 2023.
Click Here
1. CancerCell – Graduating 2023/24
Note – Accelerator can continue to support post graduate startups via convertible debt notes for sweat equity or cash contributions thus increasing share ownership position of accelerator shareholders. Original accelerator membership terms are 9% non-dilutive equity (unless anti-dilution is waived in writing) in exchange for access to accelerator mentors, advisors and shared resources with pre-emotive right for accelerator to purchase up to 20% ownership equity right up to graduation. If 9% anti-dilution clause is waived additional accelerator membership participating fees may be owed per accelerator membership agreement.
Cal-X Stars Business Accelerator, Inc. will transform into only an equity asset holding company, Cal-X Stars Holding Company, Inc. after all its startups/innovations graduate with no operations forward after last graduation. As the number of startups in portfolio dwindle down to a few the accelerator with have very heightened focus on those very few with the final graduate expected to be BioLeonhardt Whole Body our whole body regeneration chamber > https://vimeo.com/191163216
Our current bench top stimulator produced in Anaheim, California has FDA 510K K113017 Download Here
Full market clearance and CE Mark for indications of use of improving blood circulation, pain relief, improving muscle motion, arthritis treatment and joint recovery. This FDA 510K market clearance is applicable to these startups in our 2020 portfolio…AortaCell, Vascustim, PressureStim, CerebraCell, TremorStim, Second Brain, MemoryStim, Stem Cell Bra, DentaCell, OrthodontiCell, SkinStim, MyoStim ED ErectiStim, HairCell, TestiStim, ImplantStim, EyeCell, PancreaCell, KidneyCell, OrthoStim, LiverCell, EarCell, BladderCell, InStim, RegenaLung primarily based on the improving blood circulation FDA cleared indication of use and in some cases also pain relief.
PRF centrifuges and kits, adipose tissue processing kits plus devices, exosomes and regenerative fluid from amniotic sourcing all have FDA clearances for U.S. sales or research use at this time following specific labelling requirements.
Nearly all regenerative and healing product pipelines in our 2020 portfolio are divided into three related product developments following this pattern using HairCell as an example.
HairCell TM – bioelectric non-invasive treatment alone (FDA 510K)
HairCell Plus Lite TM – bioelectric + PRF and either amnio fluid or exosomes and sometimes adipose tissue derived components (FDA 510K plus lab blood bank certifications)
HairCell Plus TM – bioelectric + full stem cell and biologics composition up to fifteen components (requires BLA approval).
Both HairCell TM and HairCell Plus Lite TM may legally be commercially available now in the USA under current FDA clearances for improving blood circulation, PRF and regenerative fluid produced in certified laboratories. HairCell Plus TM bioelectric + full biologics including stem cells will require a Biologics License Approval (BLA) based on results from a well controlled pivotal study usually with > 125 treated patients and 6 to 12 months followup.
Nearly all startups in our portfolio follow this same pattern ie; MyoStim ED ErectiStim TM bioelectric only mild to moderate ED treatment, ErectiStim Plus Lite TM bioelectric + PRF and regenerative fluid for moderate to severe ED treatment, ErectiStim Plus TM bioelectric + stem cells and full biologics mix for severe advanced ED treatment. SkinStim TM bioelectric only skin treatment for mild skin regeneration, SkinStim Plus Lite TM bioelectric + PRF and regenerative fluid for moderate skin regeneration, SkinStim Plus TM bioelectric + stem cells and full biologics mix for advanced skin regeneration.
Product developments may also be further categorized as (1) non-invasive, (2) minimally invasive and (3) invasive. Non-invasive treatments are usually under FDA 510K pre-market notification clearance. Minimally invasive treatments can be covered under a combination of a FDA 510K and lab certifications while fully invasive treatment products usually require a full FDA PMA or BLA.
