EYECELL

The EyeCell eye regeneration stimulator and micro pump is designed to recover vision that may be lost from a variety of causes. The bioelectric stimulator controls release of SDF-1, IGF-1 and more than 8 other eye regeneration promoting cytokines. The micro pump is re-filled daily or weekly with our proprietary EC-15 fifteen component eye regeneration composition comprised of stem cells, growth factors, Micro RNAs, exosomes, nutrient hydrogel, anti-inflammatory agents and eye matrix. The EyeCell team is embarking on well controlled studies to seek to prove the safety and efficacy our our products for eye regeneration and vision recovery.
EyeCell has developed two products for eye regeneration:
1. Implantable micro regeneration stimulator and micro pump.
2. Non-invasive eye patch and external stimulator and pump.
Executive Summary
EyeCell is focused on the development and sales of microcurrent devices for treating eye related diseases and disorders The company has developed and patented eight product lines:
- Current of injury microcurrent devices for healing and pain management (3)
- Current of injury microcurrent bandages.
- Stem cell regeneration microcurrent device for regeneration, healing and improvement of blood flow.
- Anti-angiogenic (stops over blood supply) signaling microcurrent device.
- Stem cell growth factor cocktail compositions to be injected.
- Micropump for sustained delivery of eye repair agents over time.
- Eye health vitamins for post procedure healing.
- Microcurrent acupuncture point positioned eye patch.
The company has FDA 510K market clearance on three current of injury microcurrent products and one CE Mark for a wireless microcurrent stimulator working with selected vendors for OEM private label manufacturing.
The EyeCell microcurrent devices are designed to provide healing of injuries, reduce inflammation, reduce pain and risk of infection.
EyeCell collaborative researchers have treated nearly 3000 patients to date with 90% of them demonstrating improvement directly related to the microcurrent therapy.
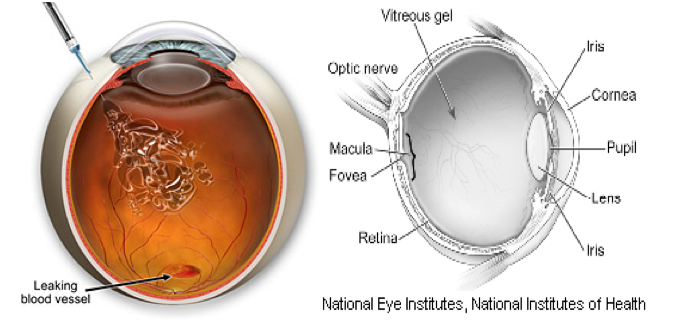
Problem – Wet Macular Degeneration = More than 20 million people worldwide suffer of wet macular degeneration related to leaky blood vessels within the eye.
Problem – Dry Macular Degeneration – Over 20 million people worldwide suffer of dry macular degeneration caused by local inflammation and oxidative stress as well as excessive accumulation of lipofuscin in the retinal pigment epitelium.
Problem – Retinal Degenerative Diseases – Over 10 million people worldwide suffer of retinal regenerative diseases –
Problem – Diabetic retinopathy – is a complication of diabetes effecting more than 20 million people worldwide that causes damage to the blood vessels of the retina—the light-sensitive tissue that lines the back part of the eye, allowing you to see fine detail. There are generally 2 causes of vision loss from diabetic retinopathy: diabetic macular edema and proliferative diabetic retinopathy.
Solution – EyeCell’s treatment armamentarium is designed to (investigational use only):
Seal off leaky blood vessels.
- Reduce inflammation.
- Regenerate tissues healing.
- Stop over blood supply.
- Increase oxygenated blood supply when needed.
- Reduce infection risk.
- 7. Reduce Pain.
Edward C. Kondrot, MD, our Executive Vice President of Clinical Studies and Co-Founder summarized three studies on the use of microcurrent for AMD:
- A two-year study (1983 to 1985) involving 114 patients, conducted by Grace Halloran, PhD. The results of the study were as follows:
- Eighteen patients had macular degeneration; 16 improved.
- Seventy-eight patients had retinitis pigmentosa; 62 showed improvement.
- Eighteen patients had other various retinopathies; 16 improved.
- Of the patients who did not demonstrate any improvement, 14 stayed the same (although they otherwise would have been expected to lose vision); two continued to lose vision, although only slightly.
- A 10-year clinical study was conducted by Drs. Jarding and Michael on the use of microcurrent to treat macular degeneration. Of the 400 eyes evaluated over the course of the study, the results were as follows:
- Seventy-eight percent of the eyes showed from 1-9 lines of improvement in reading of a visual acuity chart.
- Over 50 percent improved from 2-9 lines of improvement.
- In the study, two patients suffered from retinal vein occlusion and swelling of the macula. Both had dramatic improvement in vision.
- Damon Miller, MD, reviewed the results of using microcurrent stimulation in the treatment of Stargardt’s disease, retinitis pigmentosa and other degenerative retinal diseases. His results indicated the following:
- Of the 120 patients treated, 83 percent showed improvement of greater than or equal to two lines of visual acuity in one or both eyes.
Mechanisms of Action:
Primary = Delivers stem cells growth factors directly to wound site and stimulates the over expression of the stem cell homing signal protein SDF-1 which is also a powerful arteriogenic agen.
2. Multiples recruited and delivered stem cells to greater quantities at wound site.
3. Increases blood flow at wound site 3X (from delivering and recruiting endothelial progenitor cells and increased expression of VEGF).
4. Decreases pain at wound sites.
Strategy – 1. Add to current estate of patents. 2. Gain opinion leader support from leading institutions. 3. Get FDA AOK for specific indication of uses and reimbursement. 4. Grow to over $100 million in annual sales. 5. Auction company off to highest bidder on May 11th, 2018.
Management Team – A mix of proven medtech industry veterans and eye scientists and clinicians. Microcurrent therapy leaders.
6 focused executives on management team. Over 70 business mentors and board members
_________________________________________________________________________________________________________Business Model = Sell direct in the USA to physicians and hospitals. Sell through distributors overseas. Get opinion leaders to present data at major meetings. Attend over 20 cardiovascular meetings a year with showcase. Publish data.
_________________________________________________________________________________________________________
Competition –
Dry Macular Degeneration
Although there are still very few development projects for the treatment of dry AMD, there is a handful of projects in advanced clinical stages of development.
- Alimera Sciences glucocorticoid “Fluocinolone Acetonide” (Ph II)
- Anti-Amyloid beta antibodies from Alzheimers research of GlaxoSmithKline and Pfizer (PhII and PhI)
- Alexion pharmaceuticals C5 inhibiting antibody (Ph II)
- Acucela’s visual cycle modulator ACU 4429 (Ph II)
- Neurotech’s implant with genetically modified human cells (Ph II)
- Stem Cell approaches by ACT (embryonic) and Stem Cells Inc. (central nervous system stem cells) (Ph I / II)
- MacuClear’s and Succampos drugs for the improvement of coroidal blood flow (Ph II / III
Wet Macular Degeneration
- Lucintis from Genentech Novartis
Note – Investment in this startup is only possible through the Cal-X Stars Business Accelerator, Inc. 506c Offering at this time. Please read completely the Private Placement Memorandum (PPM) via the link below and see all the warnings posted below and within the PPM. This offering is limited to accredited investors with > $1 million in assets or income > $200K a year or $300K as a couple past two years in a row.
ALL Cal-X Stars Business Accelerator, Inc. investors must verify their accredited status via Crowdentials, EarlyIQ or Healthiosxchange.
CIRCULATION on microcurrent improvement of blood flow – Click Here
Market for dry age-related macular degeneration
- The current number of patients in the US and Europe is estimated to amount to 20 million.
- Aging populations in the western world will lead to a significant increase in numbers. In 2020,about 30 million people are predicted to suffer from dry AMD.
- The number of patients with wet AMD is comparable with the number of patients suffering from advanced dry AMD.
Related Scientific Articles
> Our patented microcurrent signal causes tissues to release SDF-1 the universal stem cell homing signal (investigational use only).
> Working with our partner in Florida, Bioheart, Inc. (founded by Leonhardt Ventures 1999), we are able to harvest stem cells from fat tissue at the clinic with a kit OR back in their GLP certified lab –http://www.bioheartinc.com/assets/press/FDAregistration.pdf – in Sunrise, Florida with expansion of quantity by cell culturing (investigational use only – training courses are available) – EyeCell is developing a series of clinical trials for utllizing of stem cells and endothelial progenitor cells combined with microcurrent stimulation for the treatment eye diseases such as wet and dry macular degeneration and seeks co-investigators and researchers to join us.
Stem cell trial sets sight on blindness
Transcorneal electrical stimulation rescues axotomized retinal … – NCBI
www.ncbi.nlm.nih.gov/pubmed/15914636
by T Morimoto – 2005 – Cited by 108 – Related articles
Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF–1 system. Morimoto T(1), Miyoshi T, Matsuda …
Electrical stimulation modulates IGF binding protein transcript levels in …
www.ncbi.nlm.nih.gov/pubmed/15584093
by S Bayol – 2005 – Cited by 20 – Related articles
Cell Biochem Funct. 2005 Sep-Oct;23(5):361-5. Electrical stimulation modulates IGF binding protein transcript levels in C2C12 myotubes. Bayol S(1), Brownson …
Effect of electrical stimulation on IGF-1 transcription by L … – Springer
link.springer.com/article/10.1007%2Fs10384-008-0533-y
by T Sato – 2008 – Cited by 29 – Related articles
Jul 27, 2008 – To investigate the effect of electrical stimulation (ES) on the induction of insulin-like growth factor 1 (IGF–1) in cultured retinal Müller cells.
IGF-1 Controlled Release by Bioelectric Stimulation Provisional Patent …
mysocialgoodnews.com/igf–1-controlled-release-bioelectric–stimulation-provisional-p…
Apr 2, 2016 – social entrepreneurship, impact investing, philanthropy … The startups via LeonhardtVentures have filed a provisional patent application with … Dr. Genovese has been conductingelectrical stimulation based regeneration …. Provisional Patent Application | My Social Good News on Howard Leonhardt to .
SDF-1 STEM CELL HOMING PROPERTIES
Low current electrical stimulation upregulates cytokine expression in …
www.ncbi.nlm.nih.gov/…/22006493
National Center for Biotechnology Information
by L Salcedo – 2012 – Cited by 4 – Related articles
Oct 18, 2011 – Low current electrical stimulation upregulates cytokine expression in the … After 1-h stimulation and euthanasia 24 h after, SDF–1 and MCP-3 …
SDF-1 Effect on Hematopoietic Progenitor and Stem Cell Mobilization …
https://www.rndsystems.com/…/sdf–1-effect-hematopoietic-progenitor-a…
R&D Systems
SDF–1 plays a key role in providing these directional cues, orchestrating CD34+ cell migration andhoming from the fetal liver into the BM.4,6-8 Both SDF–19 and …
How do stem cells find their way home? | Blood Journal
www.bloodjournal.org/content/106/6/1901
by T Lapidot – 2005 – Cited by 894 – Related articles
Homing is thought to be a coordinated, multistep process, which involves signaling by stromal-derived factor 1 (SDF–1) and stem cell factor (SCF), activation of …
SDF-1 EYE REGENERATION PROPERTIES
Stromal Cell-Derived Factor-1 Is Essential for Photoreceptor …
National Center for Biotechnology Information
by H Otsuka – 2010 – Cited by 23 – Related articles
SDF–1 blockade with a neutralizing antibody increased photoreceptor cell loss and … cells to neovascularization andregeneration sites in heart, liver,, and eye., … In some eyes, immediately after RD induction, 1 μg anti-SDF-1α antibody ..
CXCL12/SDF-1 facilitates optic nerve regeneration. – NCBI
National Center for Biotechnology Information
by A Heskamp – 2013 – Cited by 16 – Related articles
Neurobiol Dis. 2013 Jul;55:76-86. doi: 10.1016/j.nbd.2013.04.001. Epub 2013 Apr 8. CXCL12/SDF–1facilitates optic nerve regeneration. Heskamp A(1) .
IGF-1
Induction of retinal regeneration in vivo by growth factors. – NCBI
www.ncbi.nlm.nih.gov/pubmed/1936569 National Center for Biotechnology Information
by CM Park – 1991 – Cited by 115 – Related articles
Induction of retinal regeneration in vivo by growth factors. … Polymer implants containing bFGF were inserted into eyes of chicken embryos immediately … factor-beta, transforming growth factor-beta 1,insulin, or insulin–like growth factors I or II.
Effect of insulin-like growth factor-1 on corneal surface ultrastructure …
www.ncbi.nlm.nih.gov/…/24211688 National Center for Biotechnology Information
by C Wang – 2014 – Cited by 4 – Related articles
Nov 7, 2013 – Effect of insulin–like growth factor-1 on corneal surface ultrastructure and nerve regeneration of rabbit eyes after laser in situ keratomileusis. … IGF-1 can effectively accelerate the earlyrepair of corneal surface ultrastructure …
Norrin mediates angiogenic properties via the induction of insulin-like …
www.ncbi.nlm.nih.gov/…/26706283
National Center for Biotechnology Information
by LF Zeilbeck – 2015 – Related articles
Dec 17, 2015 – (2)Laboratory for Experimental Immunology of the Eye, Department of Ophthalmology, … Moreover, Norrin induces vascular repair following an … Since insulin–like growth factor (IGF)-1 is a very potent angiogenic molecule, …
HGF
Hepatocyte Growth Factor Promotes Long‐Term Survival …
https://www.deepdyve.com/…/hepatocyte–growth–factor-promotes-long-t…
Jun 4, 2014 – Read “Hepatocyte Growth Factor Promotes Long‐Term Survival and … Axonal Regeneration of Retinal Ganglion Cells after Optic Nerve … eye was performed either immediately after injury or delayed until 7 days post-injury.
FOLLISTATIN
Tissue absence initiates regeneration through Follistatin …
elifesciences.org/content/2/e00247
eLife
by MA Gaviño – 2013 – Cited by 16 – Related articles
Sep 10, 2013 – Tissue absence initiates regeneration throughFollistatin-mediated inhibition of … (D) fst(RNAi) animals lackedeye progenitors following head …
EYE REGENERATION GENERAL
An Eye on Regeneration – NCBI – National Institutes of Health
National Center for Biotechnology Information
by MK Call – 2005 – Cited by 11 – Related articles
Lens regeneration in newts is a remarkable process, whereby a lost tissue is replaced by transdifferentiation of adult tissues that only a few organisms possess.
Regenerating Lost Vision in Glaucoma | Glaucoma …
www.glaucoma.org/…/regenerating-lost-…
Glaucoma Research Foundation
Feb 10, 2016 – When we catch glaucoma early enough, we are fortunate in much of the developed world to be able to lower eyepressure with medicine or …
How fish can regenerate eye injuries at the cellular level — ScienceDaily
https://www.sciencedaily.com/releases/2016/05/160506100241.htm
Science Daily
May 6, 2016 – Scientists have examined the key function in the process of regeneration in the eyes of fish. Surprisingly, a single genetic factor triggers two …
Drug ‘cocktail’ could restore vision in optic nerve injury – Vector
vector.childrenshospital.org/…/drug-cocktail-could-restore-vision-in-optic-nerve-injur…
Jan 14, 2016 – Gene therapy achieved extensive optic nerve regeneration, … key growth factors(osteopontin, insulin–like growth factor 1 and ciliary neurotrophic factor. … But the axons weren’t able to carry signals all the way from the eye to …
VEGF and eNOS
VEGF-B can regenerate damaged peripheral nerves without …
www.news-medical.net/…/VEGF-B-can-regenerate-damaged-peripheral-…
Nov 18, 2014 – But if VEGF-B was delivered to the corneas of these mice, nerve regeneration improved. The new nerves restored normal sensation to the eye, …
Growth factor regenerates damaged nerves without …
https://news.uic.edu/growth-factor-regen…
University of Illinois at Chicago
Nov 17, 2014 – VEGF-A is a factor that Rosenblatt and several others have studied … But if VEGF-B was delivered to the corneas of these mice, nerve regeneration improved. The new nerves restored normal sensation to the eye, and proper …
Endogenous VEGF Is Required for Visual Function …
journals.plos.org/plosone/article?id=10.1371/journal.pone.0003554
by M Saint-Geniez – 2008 – Cited by 332 – Related articles
Nov 3, 2008 – To examine VEGF expression patterns in adulteye, we used mice …… of retinal ganglion cell development, survival, and regeneration.
ACTIVIN A+B
Neural Regeneration and Cell Replacement: a view from the eye – NCBI
www.ncbi.nlm.nih.gov/…/PMC26922…
National Center for Biotechnology Information
by D Lamba – 2008 – Cited by 119 – Related articles
Jun 5, 2008 – Studies of eye regeneration have a long history, dating back to the … B. The retina first becomes recognizable at the optic vesicle stage of development. …. stimulate regeneration (Spence et al., 2007), while activin signaling …
STEM CELLS
Stem cells in retinal regeneration: past, present and future
National Center for Biotechnology Information
by CM Ramsden – 2013 – Cited by 101 – Related articles
Retinal disease candidates for stem cell-based regeneration … The eye is easily accessible and there is a wealth of surgical expertise in dealing with the …. In the case of wet AMD, intravitreal injection of anti–VEGF agents can stabilise the …
Stem cells regenerate human lens after cataract surgery, restoring …
https://www.sciencedaily.com/releases/2016/03/160309135653.htm
Science Daily
Mar 9, 2016 – In the case of the human eye, lens epithelial stem cells or LECs generate replacementlens cells throughout a person’s life, though production …
A blind woman has regained sight following a controversial stem cell …
www.sciencealert.com/a-blind-woman-has-regained-sight-thanks-to-a-controversial-st…
Feb 29, 2016 – The doctor who performed the stem cell treatment, ophthalmologist Jeffrey … a case study on Belton in the journal Neural Regeneration Research. … stem cells were injected into her righteye’s retina and left eye’s optic nerve?
MICRO PUMP
Implanted Ophthalmic Micropump – Enhanced Vision
https://www.enhancedvision.com/…vision…/implanted-ophthalmic-micro…
Anyone who gets frequent eye injections for wet AMD or diabetic macular edema would be quick to agree that if there was a treatment that made it possible to …
BioElectric and Microcurrent Stimulation
Microcurrent Stimulation | Ophthamologist – Eye Diseases …
www.healingtheeye.com/microcurrent.html
Many patients become interested in microcurrent after reading my book Microcurrent Stimulation: Miracle Eye Cure and they expect to experience the same type …
Search Results
[PDF]
Electrical Stimulation to Enhance Axonal Regeneration after … – Utah
content.lib.utah.edu/utils/getfile/collection/ehsl-nam/id/1201/filename/image
Introduction: The inability of CNS axons to regenerate after injury is attributable both to … Electrical stimulation of the optic nerve elevates cAMP levels … inhibitor isobutyl methylxanthine was injected into a subset of these eyes post-stimulation.
Axonal regeneration induced by repetitive electrical stimulation of …
www.ncbi.nlm.nih.gov/…/19484445
National Center for Biotechnology Information
by Y Tagami – 2009 – Cited by 33 – Related articles
May 31, 2009 – Axonal regeneration induced by repetitive electrical stimulation of … from a corneal contact lens electrode, was used to stimulate the eye.
Therapeutic Electrical Stimulation helps to improve vision without …
https://www.restore-vision.com/new-therapy-restore-vision/
Impaired or poor vision can be improved or stabilized within few weeks by using non invasive therapeutic electrical stimulation.
First-ever restoration of vision achieved in mice | News Center …
Jul 11, 2016 – In experiments in mice described in a study published online July 11 in Nature Neuroscience, the scientists coaxed optic-nerve cables, responsible for conveying visual information from the eye to the brain, into regenerating after they had been completely severed, and found that they could retrace their …
Endogenous bioelectric currents promote differentiation of the …
Aug 30, 2017 – An EF also promoted the expression of β‐crystallin, aquaporin‐0 (AQP0) and the Beaded Filament Structural Protein 2 (BFSP2) in lens epithelial cells (LECs), all of which are hallmarks of differentiation. In addition, applied EF activated the AKT and CDC2 and inhibition of AKT reduced the activation of …
A Bionic Lens Undergoing Clinical Trials Could Give You Superhuman Abilities In Two Years
Transcorneal Electrical Stimulation Rescues Axotomized Retinal …
iovs.arvojournals.org/article.aspx?articleid=2163762
Transcorneal Electrical Stimulation Rescues Axotomized Retinal Ganglion Cells by Activating Endogenous Retinal IGF-1 System …. at 4°C. The supernatants were collected, and the protein concentration was determined by the Bradford protein assay with bovine serum albumin as a standard (Bio-Rad, Hercules, CA).
Eye regeneration technique lets blind mice see the light | Science …
Regenerating Eye Tissues to Preserve and Restore Vision – Cell Press
Jun 1, 2018 – studies for stem cell-based repair, covering key eye tissues from front to back, from cornea to retina, and including
Microcurrent stimulation in the treatment of dry and wet macular …
Dec 17, 2015 – There were 25 eyes with dry age-related macular degeneration (DAMD) and six eyes with wet age-related macular degeneration (WAMD).
(PDF) Microcurrent stimulation in the treatment of dry and wet macular …
Related Scientific Articles
Presented by Grace Halloran, Ph.D. to the
Fourth Annual Symposium on Biologically Closed Electrical Circuits, October 27, 1997, Sponsored by Mankato University, Minnesota
Abstract
From December 1995 to September 1997, thirty individuals diagnosed with typically untreatable eye diseases including retinitis pigmentosa, macular degeneration, CMV-retinitis, Stargardt disease and others attended an integrated treatment protocol employing bioelectrical stimulation, nutritional and herbal supplementation (including Ginkgo Biloba, Lutein, DHA) and other health care modalities. The study was monitored by a neuro-opthalmologist, evaluating standard clinical visual function examinations, including objective field of vision tests obtained by the Humphrey FOV analyzer, visual acuity and color discrimination. Four controls were evaluated, with the monitors masked, and although the sample was small, the results were significant in their lack of change.
DOWNLOAD• The securities may be sold only to accredited investors, which for natural persons, are investors who meet certain minimum annual income or net worth thresholds; > $200K individual income or $300K joint. > $1 million in assets excluding primary residence.
• The securities are being offered in reliance on an exemption from the registration requirements of the Securities Act and are not required to comply with specific disclosure requirements that apply to registration under the Securities Act;
• The Commission has not passed upon the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials;
• The securities are subject to legal restrictions on transfer and resale and investors should not assume they will be able to resell their securities; and
• Investing in securities involves substantial high risk, and investors should be able to bear the loss of their investment
RISK WARNING: The Cal-X Stars Business Accelerator, Inc. portfolio of innovations and startup companies are all early stage. The investment risk is very high and investors should be in position to lose all their investment without hurting their financial security. This 506c offering is limited to accredited investors only with > $1 million in assets excluding their primary residence. The offering will be posted on authorized accredited investor only portal sites that verify accreditation qualification such as www.crowdfunder.com, www.healthiosxchange.com, www.equitynet.com and www.angelist.co These portals and other outside service providers assist in verifying with reasonable assurance the accredited status of any potential investor. Merriman Capital http://www.merrimanco.com/ has been hired as an advisory registered broker dealer for this offering. Any potential investor should review all risks published within our private placement memorandum that is available upon written request to email hleonhardt@aol.com or via the above mentioned password protected portals.
Note – New 506c regulations allow general advertising and up to 2000 shareholders while remaining private – http://www.sec.gov/rules/final/2013/33-9415.pdf
IMPORTANT LEGENDS (ANY POTENTIAL INVESTOR PLEASE READ)
Securities offered under Rule 506(c) may be purchased only by accredited investors = persons with > $1 million in assets excluding their home and vehicles or whom have income > $200,000 the past two years consecutively or > $300,000 income as a couple. Accreditation status must be verified via documentation of credible third parties in a position to provide accurate verification. Investors in this offering should have experience in making early stage investments.
Cal-X Stars Business Accelerator, Inc. develops early stage innovations and startups. By nature the risk is very high for these type of investments. Any investor should be fully prepared without reserve to lose all their investment. Our high focus on developing implantable devices and biologics for treating heart failure add exceptionally higher levels of risk as the products require multiple stages of clinical trials costing many millions of dollars and require high intellectual property protection to succeed commercially. The patent landscape in these areas of participation are wrought with potential for litigation. A high number of clinical trials in the heart failure space fail to prove greater safety and efficacy compared to currently available choices. A high number of early seed stage innovations fail to ever make it to market and even a smaller number become commercial successes. This type of investment is NOT appropriate for nest egg savings.
• The securities are being offered in reliance on an exemption from the registration requirements of the 150Securities Act and are not required to comply with specific disclosure requirements under the Securities Act; the Commission has not passed upon the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials; the securities are subject to legal restrictions on transfer and resale and investors should not assume they will be able to resell their securities; and investing in securities involves risk and purchasers should be able to bear the loss of the entire investment. Private funds would be required to include a legend informing investors that the funds are not subject to the protections of the Investment Company Act.
