Principal Investigator: Rodrigo Della Méa Plentz, PhD
Telephone: +55 (51) 991131651
Email: roplentz@yahoo.com.br
Co-Principal Investigator: Jociane Schardong, PhD
Telephone: +55 (55) 981348114
Email: joci_fisioufsm@yahoo.com.br
Location of Study: Intensive Care Unit (ICU) of the Santa Clara Hospital and Pavilhão Pereira Filho of Irmandade Santa Casa de Misericórdia de Porto Alegre
Sponsor: ?
Study Design: Randomized controlled trial
Number of Subjects: 20 (The sample size can be changed)
Duration of Study: 2 to 3 weeks
Stimulation Frequency: Once a day; from admission to discharge from the ICU
Number of sessions: According to the number of days the patient remains in the ICU (on average 15-21 days)
Duration of Stimulation: Protocol 1: 30 minutes (sensory stimulation – kidneys); Protocol 2: 15 to 30 minutes (motor stimulation – quadriceps muscle)
Simulator to be Used: FDA 510K approved Mettler model 240 or similar Brazilian
Protocol: Firstly, the patients allocated to the intervention group will receive the protocol 1 and after removal of the muscle blocker they will receive the protocol 1+2. The control group will not receive any intervention, only ICU routine physiotherapy.
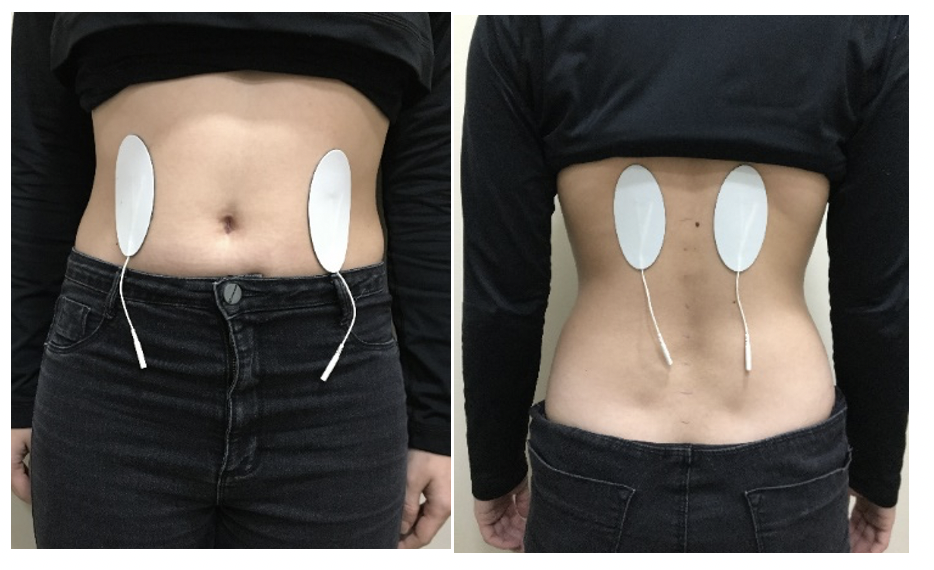
Protocol 1: For direct stimulation of the kidneys and alpha klotho protein the electrodes will be placed in the abdominal corresponding to the kidney anatomical site and dorsal region at the level of the 10th thoracic vertebra as the figure 1.
Figure 1
The parameters used will be:
- Current: symmetrical biphasic pulsed (TENS)
- Frequency: 20 Hz
- Pulse width: 1000 microseconds
- Intensity: it will be increase progressively (every session) until reaching the limit of the sensory threshold.
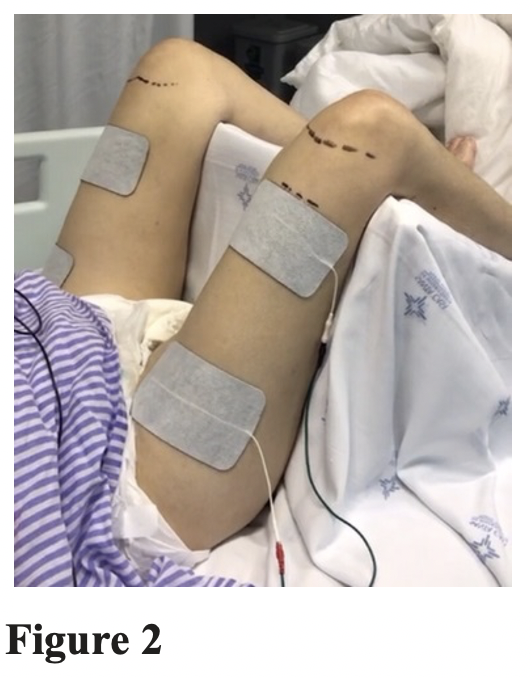
Protocol 2: To quadriceps muscle stimulation the electrodes will be placed on the motor point of the quadriceps muscle and the distal electrode was placed perpendicular to the longitudinal axis of the thigh and above the upper border of the patella in both lower limbs.
The parameters used will be:
- Current: symmetrical biphasic pulsed
- Frequency: 80 Hz
- Pulse width: 400 microseconds
- Rise: 1s
- Contraction time (ON): 10 seconds
- Decay: 1s
- Rest time (OFF): 50-20 seconds
- Mode: reciprocal
- Intensity: it will be individually adjusted to the patient’s tolerance limit to produce visible muscle contraction (motor threshold).
The detailed protocol is presented in the table 1 and the intervention for quadriceps in figure 2.
Table 1
| Week | Time of session | Contraction-Rest
ON-OFF |
Number of contractions |
| 1 | 15 min | 10-50 | 15 |
| 2 | 20 min | 10-40 | 24 |
| 3 | 20 min | 10-30 | 30 |
| 4 | 25 min | 10-30 | 37 |
| 5 | 25 min | 10-20 | 50 |
| 6 | 30 min | 10-20 | 60 |
Figure 2
Eligibility Criteria:
- Age 18-80 years old;
- Diagnosis of COVID-19
- In mechanical ventilation
- Acute respiratory distress syndrome
- Using muscle blocker at the first moment
Exclusion Criteria
- Patients who have an important sensitivity alteration
- Epidermal lesions at the application site
- Patients with pulmonary thromboembolism and thrombophlebitis
- Patient with pacemaker
- Patients with cardiac arrhythmia
- Patients with hemodynamic instability (MAP <60mmHg)
- Patients with femoral venous access (Permcath catheter);
- Patients with an intra-aortic balloon;
- Obese patients (BMI≥30);
- Patients on continuous dialysis;
- Feverish state
- Patients with epilepsy
- Pregnant
Primary Outcomes: Kidney function and systemic inflammation by α-klotho protein expression, creatinine, IL-2, IL-6, IL-10, TNFα and C-reactive protein dosage. [Time Frame: Baseline and weekly, until discharge from the ICU or death].
Secondary Outcomes:
- Muscle damage assessed by creatine kinase (CK) dosage. [Time Frame: Baseline and weekly, until discharge from the ICU or death].
- Functionality assessed using the scale Perme Intensive Care Unit Mobility Score. [Time Frame: Baseline and weekly, until discharge from the ICU or death].
- Lower limb muscle strength through scale Medical Research Council (MRC). [Time Frame: After withdrawal of sedation weekly until discharge from the ICU or death].
- Força de preensão manual por dinamometria (Hangrip): [Time Frame: After withdrawal of sedation weekly until discharge from the ICU or death].